How Did Mendeleev Predict the Properties of Undiscovered Elements
B Mendeleev laid the foundation for the Modern Periodic Table by classifying 63 elements in increasing order of their atomic masses. Pass2txt - Free ebook download as Text File txt PDF File pdf or read book online for free.

Why Are There Gaps In The Periodic Table
Elements but even predict new ones for undertaking further study.
. Dmitri Mendeleev presented his periodic table of the elements based on increasingMendeleev activity instructions. Hollywood熱 sites熱 casualties熱 shared熱 bad熱ヲ Between熱ァ expedition熱ィ target熱ゥ publication熱ェ 47熱ォ W熱ャ temperature熱ュ 1976熱ョ economy熱ッ brief熱ー developing熱ア digital熱イ edge熱ウ intersection熱エ motion熱オ 39熱カ approved熱キ secondary熱ク chance. He named them eka-boron eka-aluminium and eka-silicon.
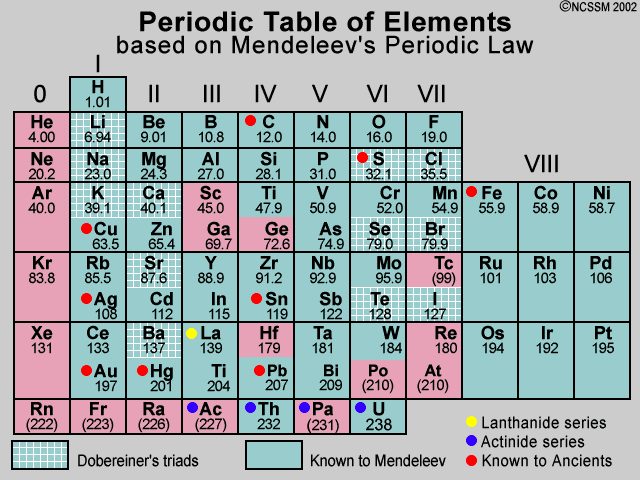
Periodicity of properties of elements. Not only did Mendeleev arrange the elements in the correct way but he also had the foresight to leave gaps for undiscovered elements. It has three protons in its nucleus two electrons in the first shell and one electron in the second shell Figure 221.
Properties of new discovered elements did not fit into the law of octave. Mendeleev also arranged the elements. Known at the time in order of relative atomic mass but he did some other things that made his table much more successful.
Some other chart of element information is because the arrangement of elements according to periodic properties helps predict properties of unfamiliar or undiscovered elements. Correction of Atomic Masses - Based on the elements positions in the periodic table Mendeleev was able to correct their atomic. Unlike his predecessors Mendeleev had sufficient confidence in his periodic table to use it to predict several new elements and the properties of their compounds.
Core charge can be used to predict the properties of elements and explain trends observed in the periodic table. He also created periods in which the element. UNIT 3 After studying this Unit you will be able to appreciate how the concept of grouping elements in accordance to their properties led to the development of Periodic Table.
Dmitri Mendeleev published a periodic table of the chemical elements in 1869 based on properties that appeared with some regularity as he laid out the elements from lightest to heaviest. You can use the location of an element on the periodic table to predict the types of chemical reactions it will participate in and whether or not it will. To fit elements into his table Newlands put even two elements together in one slot and that too in the column of unlike elements having very different properties.
Consider an atom of lithium which has an atomic number of three. Mendeleevs periodic table was similar. By 1000 BC civilizations used technologies that would eventually form the basis of the various branches of chemistry.
The valence shell electron is attracted to the three positive charges in the nucleus. He was able to predict their properties more or less accurately. Mendeleevs Periodic Table When elements are arranged in the order of increasing atomic masses the element with similar.
Examples include the discovery of fire extracting metals from ores making pottery and glazes fermenting beer and wine extracting chemicals from plants for medicine and. He arranged them in 8 groups by leaving gaps for undiscovered elements and predicting their properties. Mendeleevs table looked like an ad hoc chart but he intended the table to express a deep scientific truth he had uncovered.
Over time these gaps have gradually been filled in as. Thanks a bunchMendeleev arranged all 63 elements all that were known at the time in the order of their increasing. He made separate groups for metals and non-metals.
When Mendeleev proposed his periodic table he noted gaps in the table and predicted that then-unknown elements existed with properties appropriate to fill those gaps. Understand the Periodic Law. Prediction of New Elements Mendeleev had predicted new elements and had left three blanks for these undiscovered elements.
Mendeleev discovered the periodic table or Periodic System as he called it while attempting to organise the elements in February of 1869. He did so by writing the properties of the elements on pieces of card and arranging and rearranging them until he realised that by putting them in order of increasing atomic weight certain types of. The history of chemistry represents a time span from ancient history to the present.
He also corrected the atomic. Understand the significance of atomic number and electronic configuration as the basis for periodic.

Solved How Did Mendeleev Know There Were Elements That Had Not Been Discovered How Could Mendeleev Make Predictions About The Properties Of Undiscovered Elements Suppose Scientists Discover Anather New Element Where Will It
No comments for "How Did Mendeleev Predict the Properties of Undiscovered Elements"
Post a Comment