2. Describe Polar Covalent Bonds Using Water as an Example
Dipole moment is used to calculate the percentage ionic character of a covalent bond. - increases across the periodic table from right to left and bottom to top.
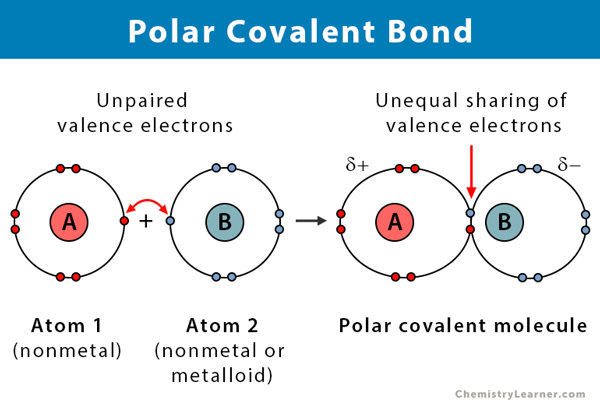
Polar Covalent Bond Definition And Examples
The more electronegative atom is said to have a partial negative charge and the less electronegative atom has a.
. Examples of molecular compounds that dissolve well in water are sugar and ethanol. - a covalent bond in which the electron distribution between atoms is uneven. Learn with flashcards games and more for free.
By sharing the two electrons where the shells touch each hydrogen atom can count 2 electrons in its outer shell and the oxygen atom can count 8 electrons. Polar covalent molecules dissolve well in a polar solvent such as water. H 2 O water is not a 100 covalent compound it has some ionic bond character as well.
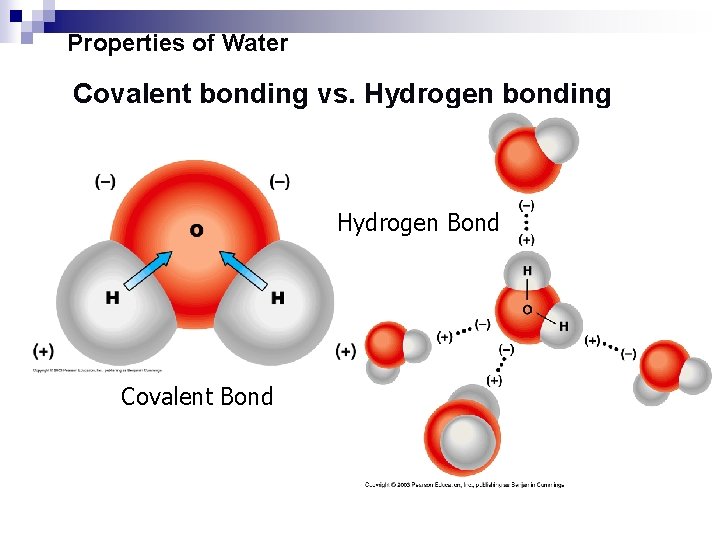
The water molecule has two polar covalent bonds between oxygen and hydrogens. Carbon dioxide being linear the net bond moment is equal to zero since the individual bond moment cancel with each other. Examples of polar covalent bonds The water H 2 O is the most classic example of a polar molecule.
Describe and differentiate between covalent and ionice bonds using examples of each. A polar covalent bond is formed when atoms which are having different electronegativities and share electrons between them. For water the structure is H-O-H.
The polar covalent character of water allows its molecules to become attracted to many other different types of molecules and disrupt their pre-existing bonds. Most covalent bonds are slightly polar in nature. Terms in this set 43 polar covalent bonds.
As electrons are always negatively charged. It is said that water is the universal solvent but this does not mean that it dissolves universally but rather that due to its abundance it is a suitable solvent to dissolve polar substances Helmenstine 2017. Polarity of Covalent Bond in Water Molecule An illustration describing the polarity of the covalent bonds in a water molecule is provided above.
In the water molecule H2O the electrons of the hydrogens stay closer and longer around the oxygen which is more electronegative. Electronegativity of H 21 electronegativity of O 35. A water molecule condensed as H2O is an example of a polar covalent bond.
Examples of molecules with polar bonds include water hydrogen fluoride sulfur dioxide and ammonia. A water molecule abbreviated as H2O is an example of a polar covalent bond. A _____ bond forms between polar molecules.
Polar bonds are intermediate between pure covalent bonds and ionic bonds. Carbon tetrachloride has zero dipole moment since the molecular is highly symmetrical with tetrahydro structure. This is due to the different electronegativity electron attracting values of the individual atoms.
However it is important to note that the bond formed between two individual water molecules is a hydrogen bond and not covalent. Lets have a look at some of them. In this type of bond one of the atoms is stronger than the other and attracts the electrons so that they spend more time closer to the stronger atom.
- the in-between step from covalent to ionic. They form when the electronegativity difference between the anion and cation is between 04 and 17. Two hydrogen atoms each share their 1 electron with oxygen to form two covalent bonds and make a water molecule H 2 O.
The electrons are unequally shared with the oxygen atom spending more time with electrons than the hydrogen atoms. Water is a polar covalent molecule. 1 A Describe a polar covalent bond and give an example.
This is a picture of a water molecule. If the electronegativity difference between the two atoms is between 05 and 20 the atoms form a polar covalent bond. The difference in electronegativity between Oxygen 34 and Hydrogen 22 is 342212 which is lower than 17 hence according to the Pauling scale the bond formed between oxygen and a hydrogen atom is covalent in nature.
B Describe a nonpolar covalent bond and give an example. In short here is the summary. A covalent bond that has an unequal sharing of electrons and the electronegativity difference is within the range 01-2 is called a polar covalent bond.
- the ability of an atom to attract electron in a covalent bond. Lets look at water H 2 0. If the electronegativity difference between the atoms is greater than 20 the bond is ionic.
Hydrogen can only form 1 bond. This is because of the unequal sharing of electrons due to the difference in electronegativity between oxygen and hydrogen. A covalent bond is a bond where atoms share electrons.
The electronegative difference between the H and O allows them to be polar covalent. A polar covalent bond is a type of bond between two or more atoms in which the atoms do not share their pair of electrons equally. An oxygen atom has six electrons.
Fortunately you can look up electronegativity on a table to predict whether or not atoms are likely to form polar covalent bonds. Since electrons consume more time with the oxygen atom it carries a partial negative charge. However nonpolar covalent.
Fluorine F is the most electronegative element 40 and has seven valence electrons. The electrons are unevenly shared with the oxygen atom using more time with electrons than the hydrogen atoms. Water is a covalent bond between.
Nitrosyl Chloride NOCl Nitric Acid HNO3 Carbon Monoxide CO Phosphorus Pentoxi de. What this means is that electrons are not evenly shared between the atoms.
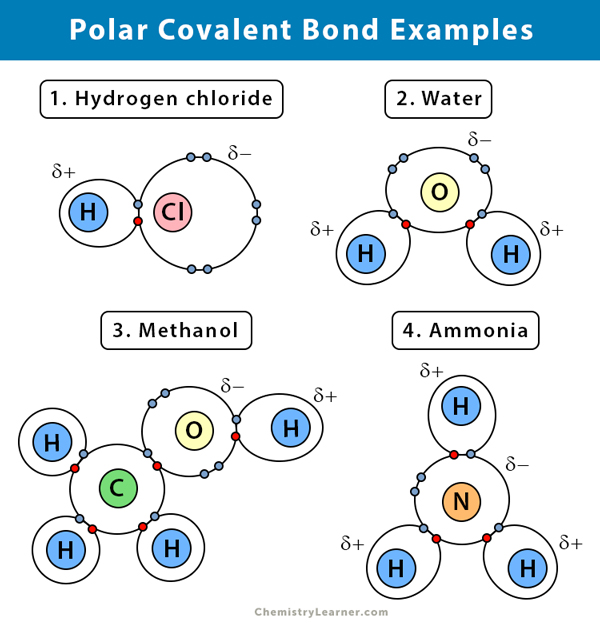
Polar Covalent Bond Definition And Examples
No comments for "2. Describe Polar Covalent Bonds Using Water as an Example"
Post a Comment